Le Chatelier's Principle Continued Worksheet
The first one is completed for you Key Questions 1. Le Chateliers Principle Worksheet 1 For the reaction below which change would cause the equilibrium to shift to the right.

Le Chatelier S Principle Example Problems
Le Chateliers Principle Name_____ CHEMISTRY.
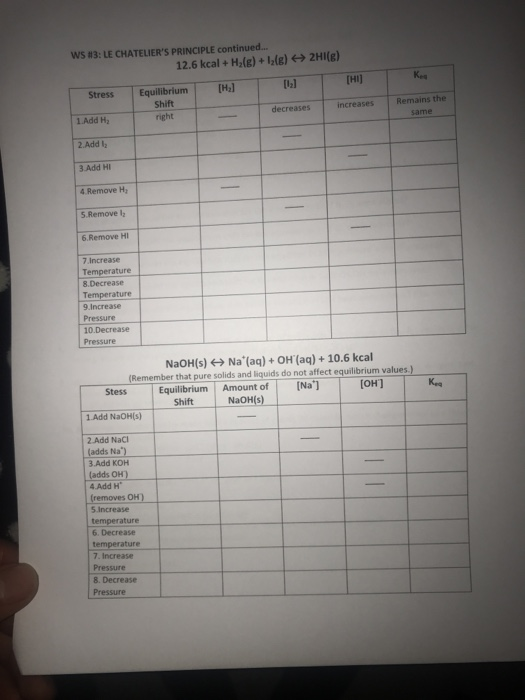
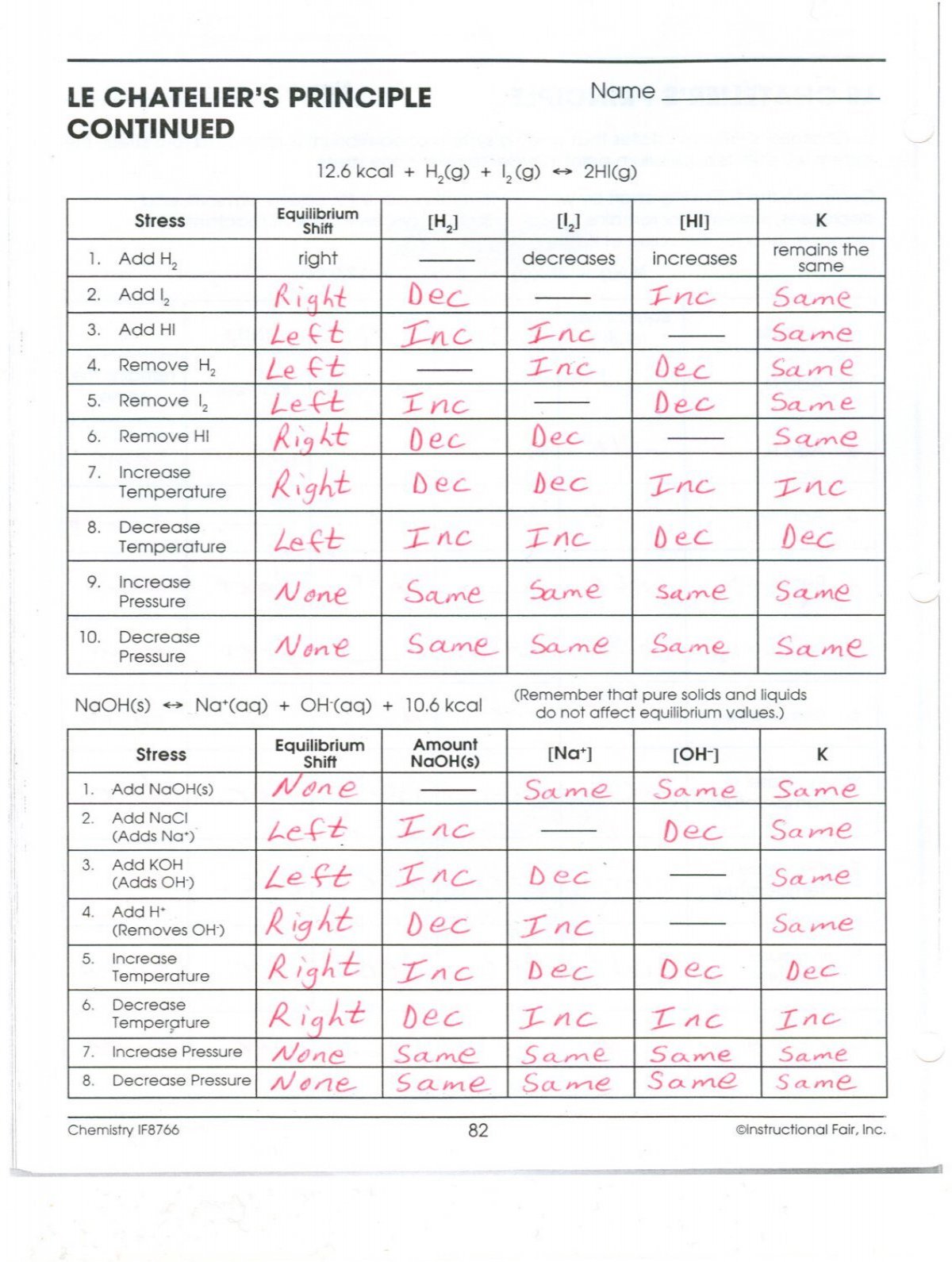
Le chatelier's principle continued worksheet. Equilibrium shifts to the right. LE CHATELIERS PRINCIPLE CONTINUED Name 2Hlg 126 kcal Equilibrium Shift right 2. Complete the following chart by writing left right or none for equilibrium shift and decreases increases or remains the.
Discover learning games guided lessons and other interactive activities for children. Discover learning games guided lessons and other interactive activities for children. A Study of Matter 2004 GPB 1211 If a system at equilibrium is subjected to a _____ the equilibrium is.
Stress Add H2 Add 12 Add HI Remove 1-12. Add KOH Adds OH- 4. Le Chateliers principle states that if the temperature is increased the equilibrium will change to decrease the temperature of the vessel.
A study of matter 2004 gpb 12 11 if a system at equilibrium is subjected to a the equilibrium is. Le chateliers principle continued worksheet answers Equilibrium Worksheet Questions Power Point Lesson Notes- double click on the lesson number. Ad Download over 20000 K-8 worksheets covering math reading social studies and more.
Add Naa Adds Na 3. Le Chateliers Principle - Practice Problems for Assignment 4 Consider the following equilibrium 1 when answering questions 1 and 2. Le Chateliers PrincipleA statement of Le Chateliers PrincipleIf a dynamic equilibrium is disturbed by changing the conditions the position of equilibrium.
Once you find your worksheet click on pop-out icon or. Some of the worksheets displayed are Le chateliers principle Le chateliers principle work Concentration temperature volume total pressure Work lechateliers principle name Work 19 Work le chateliers principle name. Ad Download over 20000 K-8 worksheets covering math reading social studies and more.
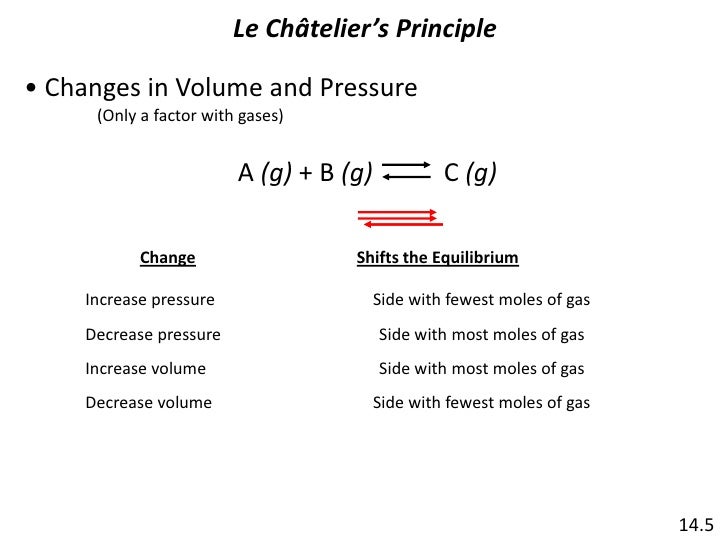
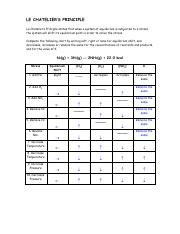
In Table 1 apply Le Chateliers Principle and indicate the direction of the shift in equilibrium if the indicated stress is applied to the reaction system. H 2O l H 2O g 1 1. Le Chateliers Principle.
Showing top 8 worksheets in the category - Le Chateliers Practice. t l j e also called Chateliers principle or the Equilibrium Law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibriaThe principle is named after French chemist Henry Louis Le Chatelier and sometimes also credited to Karl Ferdinand Braun. L t l j e or US.
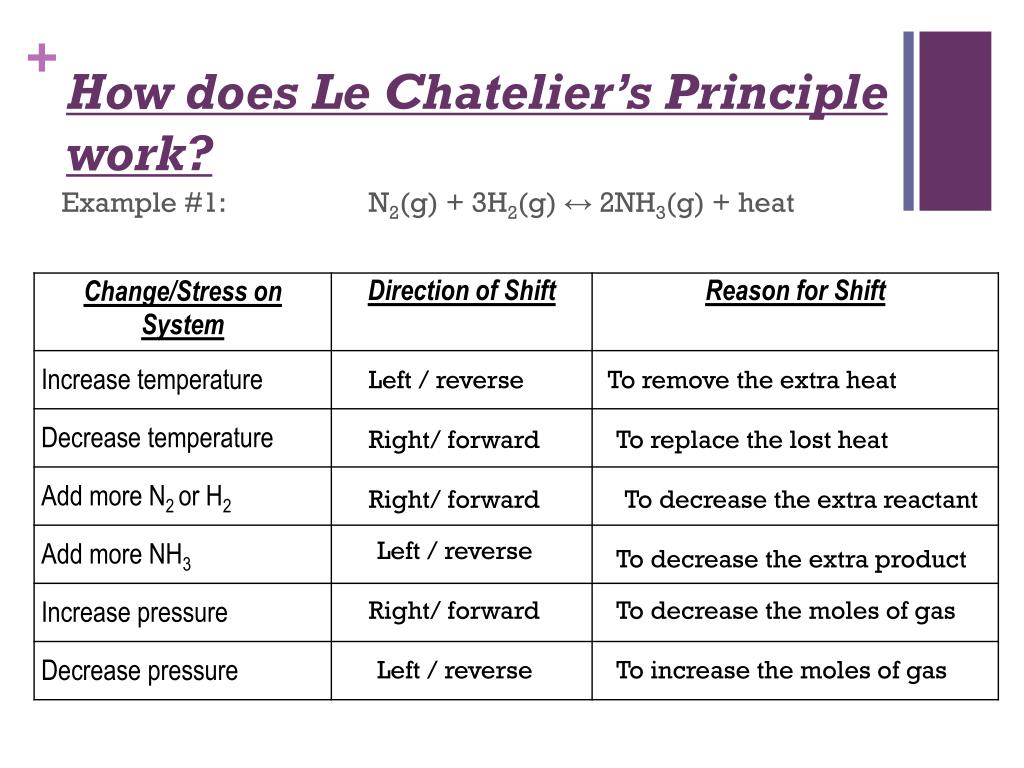
The number of moles of the reactants decreases after the temperature increase while the number of moles of the product increases. Le Chateliers Principle explains shifts in the equilibrium system due to changes in concentration temperature pressure and the addition of a catalyst and the eventual establishment of a new equilibrium. This all comes back to the idea of collision theory.
Le Chateliers principle pronounced UK. C Increase the temperature of the system. Add Removes OH 5.
Remove HI Increase Temperature 8. LE CHATELIERS PRINCIPLE CONTINUED 126 kcal H2g Name 12 g 2Hlg Stress Add H2 2. B increase the pressure on the system.
The stresses are changes in concentration pressure and temperature. Com lete the followin table. Le Chateliers Principle states that when a system at equilibrium issubjected to a stress the system will shift its equilibrium point in order to relieve the stress.
Which of the following is true about reaction 1. LeChateliers Principle-1 WS 2 3. Decrease Pressure Na S Stress Add Nao s 2.
The following video continues with the previous explanation with the picture of. That is the reaction that takes in heat endothermic will be favoured. Le Chateliers Principle and Changing Pressure or Temperature DOC 29 KB LeChateliers Principle Warm Up DOC 32 KB Le Chateliers Principle Worksheet DOC 33 KB Past Units Review DOC 28 KB Physical Equilibrium Worksheet DOC 30 KB Two gases NO2 and N2O4 are in Equilibrium Worksheet DOC 30 KB Writing Forward and Reverse Reactions.
If a stress is applied to a system at equilibrium the system will tend to adjust to a new equilibrium which minimizes the stress if possible. C increase the temperature of the system. Approaching Equilibrium WS 1 Q1 2.
Stress Concentration H2 increases Concentration 1-12 decreases Concentration of 02 increases. In 1884 Henri Le Chatelier formulated the following principle. Le chatelier principle displaying top 8 worksheets found for this concept.
B Increase the pressure on the system. Which of the. Decrease Temperature 9 Increase Pressure 10.
Worksheet le chatelier s principle. CH4g 2H2Sg CS2g 4H2g a Decrease the concentration of dihydrogen sulfide.
Ppt Catalyst Powerpoint Presentation Free Download Id 5648517

Le Chatelier S Principle Example Problems

Four Page Lab That Has Students Exploring How This Reaction Changes With Temperature And Concentr Le Chatelier S Principle Chemistry Labs High School Chemistry
Le Chatelier S Principle Example Problems
Worksheet 3 Le Chatelier S Principle Le Chatelier S Chegg Com

Effect Of The Change In Pressure On Equilibrium State Le Chateliers Prin Equilibrium Le Chatelier S Principle Chemistry Notes

Le Chatelier S Principle Example Problems

Le Chatelier U2019s Principle Le Chatelier U2019s Principle Le Chatelier U2019s Principle States That When A System At Equilibrium Is Subjected To A Stress Course Hero
Le Chatelier U2019s Principle Le Chatelier U2019s Principle Le Chatelier U2019s Principle States That When A System At Equilibrium Is Subjected To A Stress Course Hero

Https Www Pinterest Com Explore Le Chatelier S Principle Teaching Chemistry Chemistry Education Chemistry Lessons
Https Bergerchem Weebly Com Uploads 2 5 2 7 25273844 Answers Le Chateleirs Principle Ws Pdf

Le Chatelier S Principle Example Problems
Le Chatelier S Principle Example Problems

Le Chateliers Principle By Beccy597 Teaching Resources Tes Worksheet Template Tips And Reviews
Le Chateliers Principle By Beccy597 Teaching Resources Tes Worksheet Template Tips And Reviews